Explain Differences Between Ph in Lab Reasons
Bicarbonate concentration can be either normal. A ph of 7 would mean it is neutral.
Water Quality 101 What Is Ph In Water Testing
This is because the concentration of hydrogen ions and hydroxide ions are equal.

. Obtaining the correct results from a. The value of Kw Water ionisation constant and pH with increasing temperature. Acidic solutions have a pH value lower than 7 and basic or alkaline solutions have a pH level higher than 7.
The smaller the volume the greater the gravity and the darker the color. A ph of 8 to 14 would be a basic solution. Secondary - Includes the alpha helix formed by coiling of the peptide chain and a beta pleated sheet structure formed between protein strands.
Explain the relationship between the color specific gravity and the volume of the urine. An acid can donate the hydrogen ion H and the base is a substance which can accept the ion. An acidic solution has a ph ranging from 1 to 6.
Lower dissociation percentage causes the solution to have less hydrogen ion in solution resulting in a higher pH value. The experimental results are based on real time system provides much accurate results compared to simulation results. Explain two reasons why glucose might be present in urine.
The normal pH range for blood is between 735 and 745. A pH below 735 is an acidemia and a pH above 745 is an alkalemia. The pH then is the concentration of the ion in solution.
Metabolic acidosis is more severe than respiratory acidosis. This discussion intends to impart a basic understanding of acid-base balance in the body while providing a systematic way to approach patients who present with. Soil pH is important for crop germination and growth.
Both conditions are increases of acidity of blood but the places and processes are different as the names indicate. These are usually carried out as titrations so the exact amount of one reactant added to the other can be monitored. PKa Acidity is the state of being an acid.
Metabolic Acidosis vs Respiratory Acidosis. Record the color and the pH after 3 drops of NaOH have been added. PH papers will give approximate pH values whereas pH meters give more accurate values.
A solution that contains more hydroxide ions is basic. The data section should include three tables. The more hydroxide ions hydrogen oxygen the more basic it is.
Investigating pH of common household substances is designed for a 6th grade middle school classroom. This makes them useful for different reactions between acids and bases which have different equivalence points. Every solution will undergo a change in their pH value through changes in temperature.
There are pH meters and pH papers in laboratories which can be used to measure pH values directly. This is important not only in the chemistry lab but in industry cooking and medicine. High pH causes a bitter taste water pipes and water-using appliances become encrusted with deposits and it depresses the effectiveness of the disinfection of chlorine thereby causing the need for additional chlorine when pH is high.
A concentration of acid after each dilution b experimental pH of each dilution c. It shows the color of the universal indicator under different pH conditions. Metabolic acidosis has more causes than respiratory acidosis.
PH is carefully regulated in human cells and blood. If the pH is at the low end of the scale or if it is actually below 735 the condition is acidemia. The new pH level simply tells about the true pH for that solution at that specific temperature.
The most common cause of metabolic acidosis is the presence of organic acids or excessive ketones in the blood. If a persons blood pH drops below 735 then he or she is in metabolic acidosis. Context for Use.
Table 1 lists some other causes of metabolic acidosis. Primary - The peptide bonds that join one amino acid to the next. The first table should be for the acid and include the following information.
Properties of Acids Bases. Add 01 M NaOH to the sample of HCl above one drop at a time. For example phenolphthalein has a range of 83-100 and is useful for the titration of a strong acid with a.
Further the acidity decreases as the value of pH increases from 0 to 7 whereas solutions with the value of pH equal to 14 are termed as strongly basic solutions. Low-pH water will corrode or dissolve metals and other substances. For example Nofzinger 2004 used PET scanners to investigate the differences in brain metabolic activity during sleep between people with insomnia and a control.
The curve at equivalence point for Part III graph is comparatively positioned higher than those of the Part II graph because for strong acid-strong base titration pH 7 at equivalence point while for weak acid-strong base titration pH 7 at equivalence point. Water is neutral because it has one H ion and one OH- ion so they balance out and are neutral. Define pH on the board by telling students that the more reactive hydrogen in a liquid the more acidic it is.
You should use a calibration range 4. The basicity decreases as the value of pH decreases from 14 to 7. A difference in pH measurements at different temperatures is NOT an error.
A 6 M NH3 is accidentally added instead of hot water in. Explain the reason for the difference in conductivity between the two acids. As a result they give same value in buffer and different values in soil suspension I think.
Usually your body maintains the pH of your blood close to 74. You just studied 19. At the normal pH of 740 the ratio of bicarbonate to carbonic acid buffer is 201.
Solutions having the value of pH equal to 0 are known to be strongly acidic solutions. The calibration range of the pH meter is also significant fact. Blood which is over 90 water is normally slightly basic with a normal pH range of about 735 to 745.
The procedure section should reference the lab manual and include any changes made to the lab manual procedure during the lab. Laboratory experiments also allow researchers to take advantage of the new technology available to them. This type of scientific evidence could not be achieved in a field experiment.
Explain the expected results if the following errors were made during the lab. The activity is conducted as a lab with emphasis on prior determined safety rules. Normal blood pH ranges from 735 to 745 this is slightly to the alkaline side of the scale.
No tasting keep area clear of other materials place used materials on paper towel self-monitor movement between lab stations. Variation by even a tenth of a pH unit may be fatal. Due to the importance of sustaining a pH level in the needed narrow range the human body contains compensatory mechanisms.
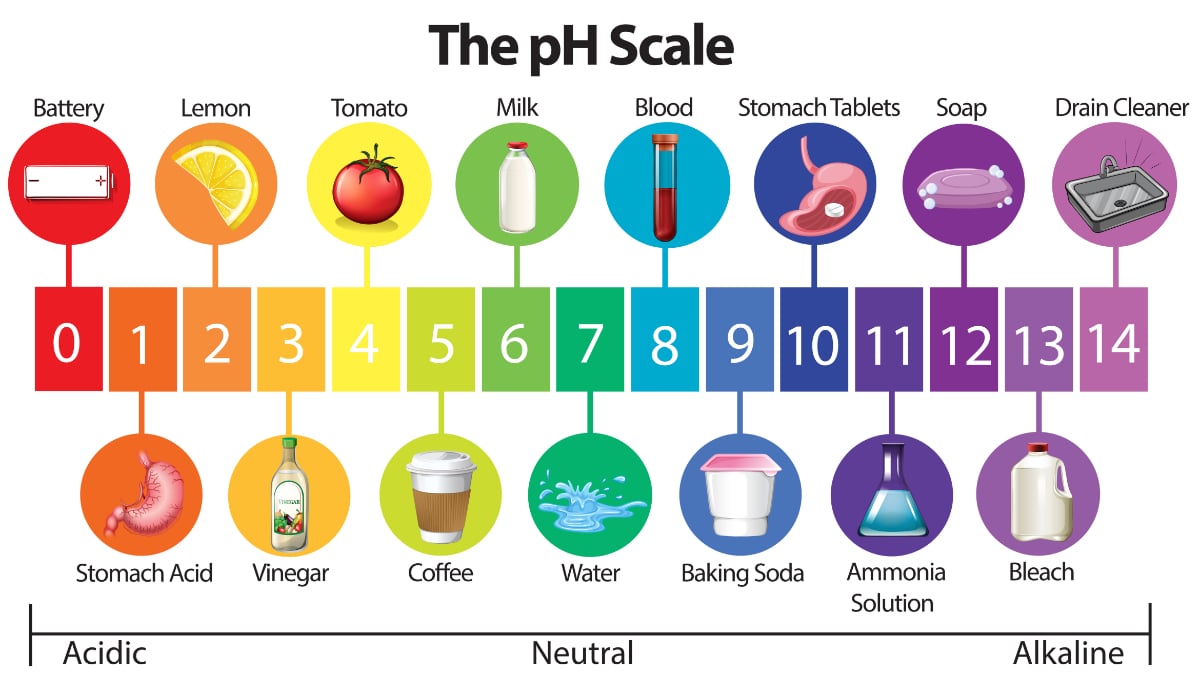
A pH level of 70 is neutral and represents the middle of the scale.

A Breakdown Of The Uses Of Ph In Different Industries

Virtual Ph Lab Explaining The Role Of Ph In Food Science Teaching Chemistry Science Skills 8th Grade Science

Difference Of Alkalosis And Acidosis Nursing School Survival Nursing School Tips Nursing School Notes
No comments for "Explain Differences Between Ph in Lab Reasons"
Post a Comment